Splitting Isotopes
By: Shaila Murthy
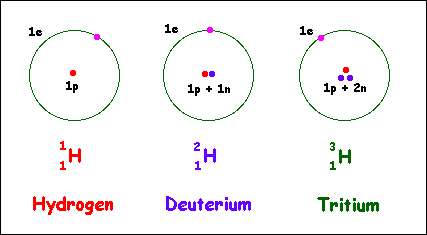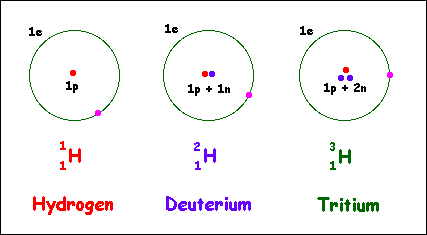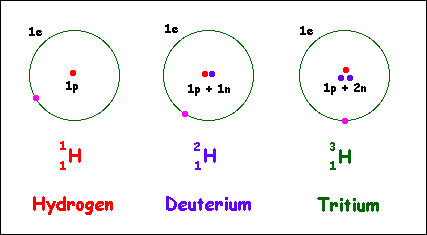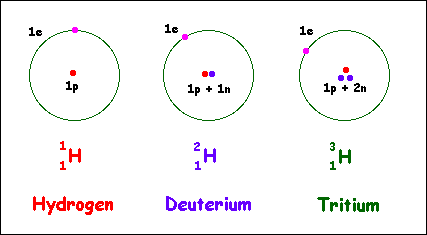
An isotope is an atom that contains an extra neutron, creating an unstable nucleus. Over time, the isotopes of various elements have been found to be useful, especially uranium and radioisotopes. While these are very helpful, they are also very hard to create, due to how difficult it is to separate them. Each isotope differs only by one our two neutrons, and this extreme similarity in mass is what makes it such a troublesome task to split them. However, some researchers from the University of Chicago have discovered a new way to split atoms into isotopes, a way that will make it a much simpler task to perform.
Professor Steven J Sibener and his team worked with neon to conduct their experiments, as well as a silicon wafer (a thin piece of semiconductor material) with a specific intricate pattern. They used a supersonic beam of neon where all of the atoms were accelerated to the same speed. That beam was then shot into the crystalline surface of the silicon wafer, and the individual beams shoot off at different angles dependent upon their isotopic composition. The precise pattern of the silicon wafer is what causes this to happen, as the atoms of the crystalline surface are lined up exactly so that the incoming atoms will hit them at just the right angles. This particular experiment resulted in the two neon isotopes 22Ne and 20Ne. The researchers also observed that the isotopes bounced off of the silicon wafer at slightly different velocities, meaning that higher levels of enrichment and purification could be obtained through this method by using velocity separation.
An isotope is an atom that contains an extra neutron, creating an unstable nucleus. Over time, the isotopes of various elements have been found to be useful, especially uranium and radioisotopes. While these are very helpful, they are also very hard to create, due to how difficult it is to separate them. Each isotope differs only by one our two neutrons, and this extreme similarity in mass is what makes it such a troublesome task to split them. However, some researchers from the University of Chicago have discovered a new way to split atoms into isotopes, a way that will make it a much simpler task to perform.
An image depicting the experiment conducted, showing the path of the neon gas cloud hitting the crystalline surface, and the two neon isotopes shooting off of it.
This process is extremely important because it could lead to great advancements in science, particularly in the medical field. Radioisotopes are extremely useful in medicine because they can be detected within the body. Thus, they can be administered into the body, and then photographed using a gamma camera. Those images are then computer enhanced and the physicians can now see blood flow, and can pinpoint where tumors and fractions are. The other popular isotopes, those of uranium, are used for their radioactive qualities, and are often converted into uranium dioxide to be used to power reactors. Nuclear weapons, however, utilize the metallic form rather than the gaseous form.
Sources:
1.) Lerner, Louise. "Chemists Introduce Novel Method to Separate Isotopes". Phys.org. Science X
Network, 24 October 2017. Web. 26 October 2017.
2.) Nihill, Kevin J., Jacob D. Graham, and Steven J. Sibener. "Separation of Isotopes in Space and Time by Gas-Surface Atomic Diffraction." APS Physics. American Physical Society, 23 Oct. 2017. Web. 26 Oct. 2017.
3.) "Isotopes." NuclearConnect. American Nuclear Society, n.d. Web. 26 Oct. 2017.
4.) "Uranium: Its Uses and Hazards." Institute for Energy and Environmental Research. Institute for Energy and Environmental Research, May 2012. Web. 26 Oct. 2017.


Comments
Post a Comment